Geron (GERN.US), US biotechnology company, is surging almost 100% today! Share price surge comes in response to US Food and Drug Administration (FDA) advisory panel approving company's anemia drug candidate, Imetelstat. FDA panel voted 12-2 in favor of the drug, saying that its benefits of Imetelstat outweigh risks for the targeted patient population. While final approval from FDA is expected by June 16, 2024, FDA often follows recommendations of advisory panels.
While FDA is not obliged to follow advisory panel recommendations, a strongly positive 12-2 vote results shows that there is a very high chance of regulatory approval in June.
Taking a look at Geron chart (GERN.US) at D1 interval, we can see that today's surge is indeed massive. Stock bounced off the $1.70 support zone and rallied to the highest level since mid-June 2023. Advance has been halted at the $3.40 resistance zone, marked with local highs from the first half of 2023. Nevertheless, even after today's surge the stock is trading over 50% below its all-time highs from September 2018.
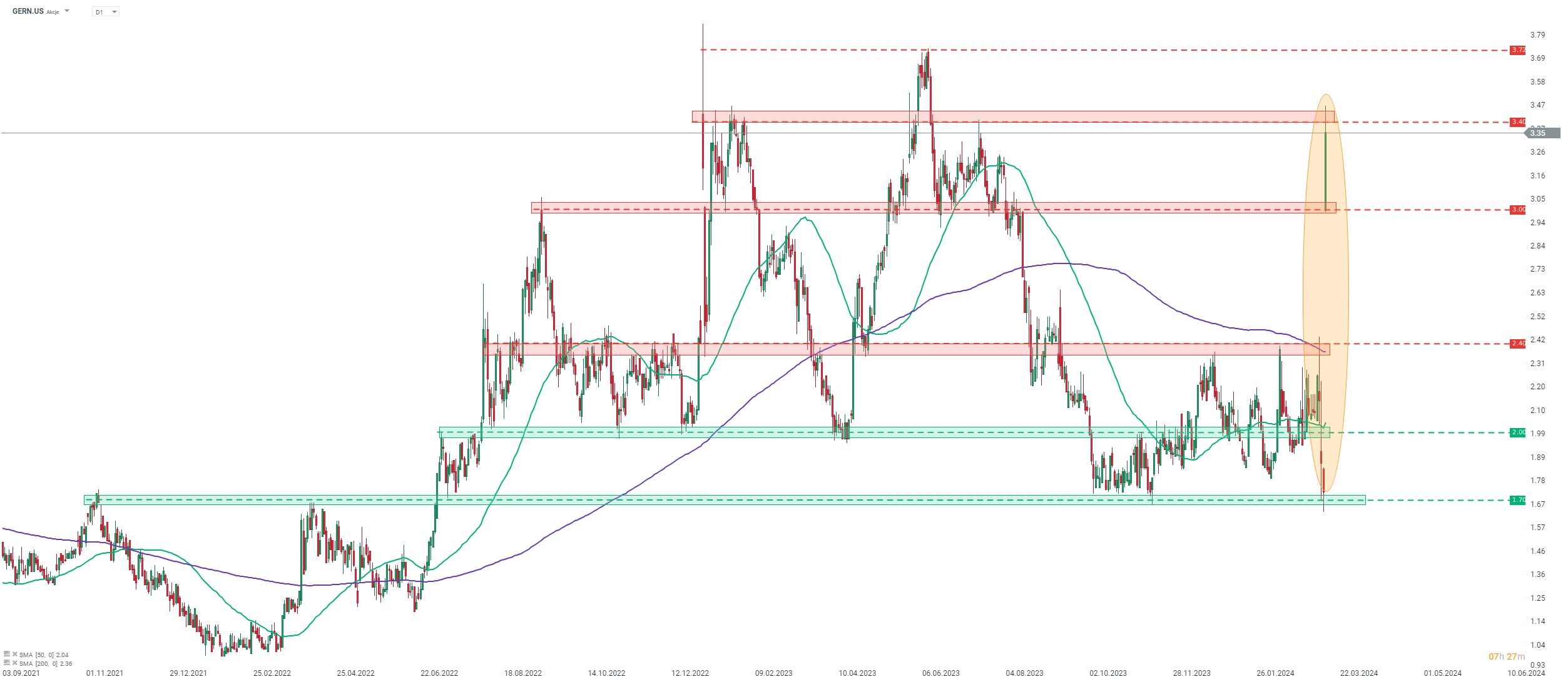 Source: xStation5
Source: xStation5

Daily summary: Weak US data drags markets down, precious metals under pressure again!
Datadog in Top Form: Record Q4 and Strong Outlook for 2026

US Open: Wall Street rises despite weak retail sales

Coca-Cola Earnings: Will the New CEO Withstand the Pressure?
This content has been created by XTB S.A. This service is provided by XTB S.A., with its registered office in Warsaw, at Prosta 67, 00-838 Warsaw, Poland, entered in the register of entrepreneurs of the National Court Register (Krajowy Rejestr Sądowy) conducted by District Court for the Capital City of Warsaw, XII Commercial Division of the National Court Register under KRS number 0000217580, REGON number 015803782 and Tax Identification Number (NIP) 527-24-43-955, with the fully paid up share capital in the amount of PLN 5.869.181,75. XTB S.A. conducts brokerage activities on the basis of the license granted by Polish Securities and Exchange Commission on 8th November 2005 No. DDM-M-4021-57-1/2005 and is supervised by Polish Supervision Authority.


