Moderna (MRNA.US) shares rose 25% today after results of a new mRNA vaccine showed promise in a preliminary study. Also gaining along with Moderna are shares of Merck (MRK.US), whose immunotherapy combined with an mRNA vaccine reduced the risk of cancer recurrence in skin cancer patients. Also gaining on the wave of news are shares of Biontech (BNTX.US), a company that, in collaboration with Pfizer (PFE.US), was the first to receive Federal Drug Administration (FDA) approval to produce an mRNA vaccine against Covid-19:
- Moderna and Merck reported that the combination of a personalized mRNA cancer vaccine and Keytruda immunotherapy from Merck reduced patients' risk of relapse or death by nearly 44%, in a study involving 150 volunteers. The use of the vaccine has increased the previously recorded efficacy of immunotherapy alone;
- If proven effective in further trials, the results could expand the use of mRNA products from Moderna beyond the Covid-19 vaccine itself, which was the first application of gene-based vaccine technology;
- According to the companies, the results were statistically significant, but still unverified by independent scientists. They also prompted a flurry of speculation around a class of vaccines gaining popularity in medicine but still not well understood enough, which aim to treat diseases rather than prevent infections, as with typical injections. Merck and Moderna reported , that they believe the immune response will complement the effects of the drug Keytruda, which works by releasing the brakes on the body's immune system to fight cancer.
The companies said they plan to conduct a larger study next year to confirm the safety and efficacy of the combination of immunotherapy and mRNA technology. Positive results from this study could pave the way for potential approval of Moderna's experimental cancer vaccine. Moderna and Merck also plan to test their combination in other types of cancer:
"We don't want to waste time. Given that the data is so strong, for me this is a Covid-like moment" said Stephane Bancel, Moderna's CEO.
- Moderna has been looking for new applications for its mRNA technology since it was approved in the widely used Covid-19 vaccine. Cancer therapies are one of the pharmaceutical industry's largest markets. Moderna manufactures its vaccine in a complex way, as the injection is customized for each patient;
- The development of a personalized cancer vaccine from Moderna was delayed by the Covid-19 pandemic, at which time patient enrollment was temporarily halted, and Moderna used some of its cancer vaccine manufacturing equipment to make early batches of its Covid-19 injections, CEO Bancel said.
- In 2019, Moderna and Merck began a second-gasp study of a personalized cancer vaccine with Keytruda in people whose melanoma had been removed by surgery, but were considered at high risk of recurrence. After surgery, some patients received up to nine doses of the personalized cancer vaccine, administered every three weeks, while receiving Keytruda every three weeks for up to 18 cycles. Another group received Keytruda alone.
"This is the first time we've seen a really strong signal with a cancer vaccine.This is the first time we can actually show that by removing the brakes on the immune response that Keytruda provides, plus an exceptionally good vaccine, we induce a really great immune response" - said Eliav Barr, head of global clinical development and chief medical officer of Merck. The two companies released key data from the study in a press release and plan to publish all available details in the peer-reviewed, professional medical press.
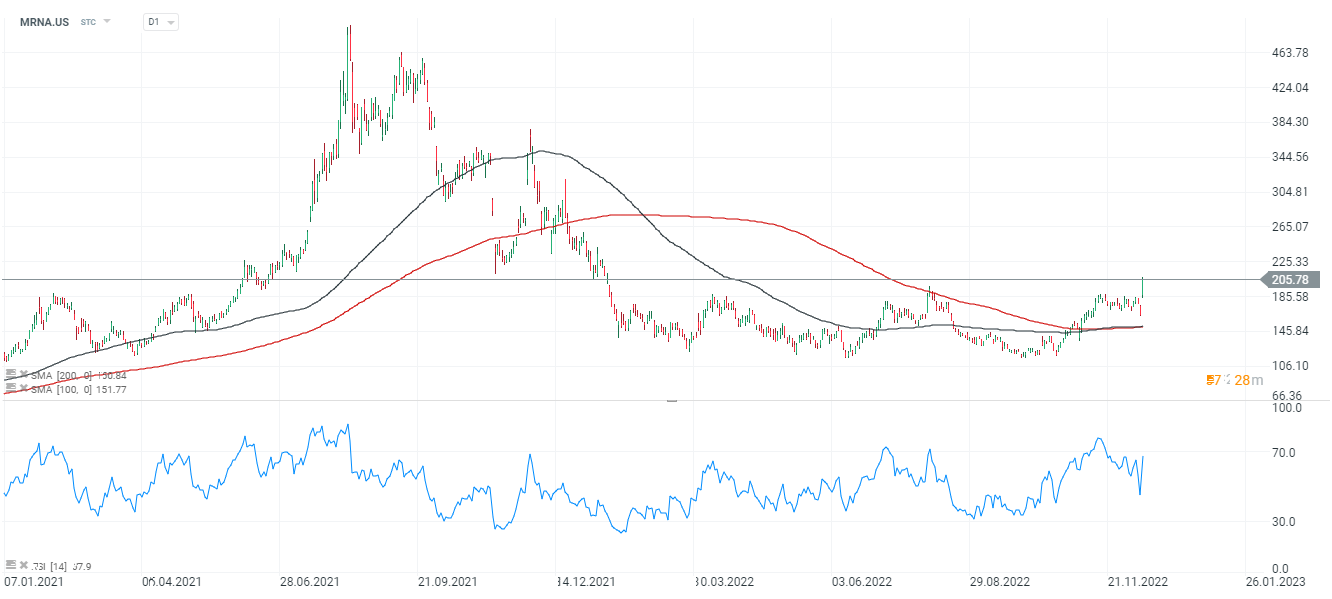 Moderna shares (MRNA.US), D1 interval. Moderna's shares reacted with powerful growth to news of a potential cancer vaccine. The SMA200 (red) and SMA100 (black) averages approached the bullish intersection known as the 'golden cross'. The stock is still trading at a nearly 60% discount to its peaks in the fall of 2021. Source: xStation5
Moderna shares (MRNA.US), D1 interval. Moderna's shares reacted with powerful growth to news of a potential cancer vaccine. The SMA200 (red) and SMA100 (black) averages approached the bullish intersection known as the 'golden cross'. The stock is still trading at a nearly 60% discount to its peaks in the fall of 2021. Source: xStation5

Gazdasági naptár: NFP-adatok és amerikai olajkészlet-jelentés 💡

Live Trading - 2026.02.10.

🌍 Gyorsjelentési szezon az XTB-vel

🌍 Gyorsjelentési szezon az XTB-vel
Ezen tartalmat az XTB S.A. készítette, amelynek székhelye Varsóban található a következő címen, Prosta 67, 00-838 Varsó, Lengyelország (KRS szám: 0000217580), és a lengyel pénzügyi hatóság (KNF) felügyeli (sz. DDM-M-4021-57-1/2005). Ezen tartalom a 2014/65/EU irányelvének, ami az Európai Parlament és a Tanács 2014. május 15-i határozata a pénzügyi eszközök piacairól , 24. cikkének (3) bekezdése , valamint a 2002/92 / EK irányelv és a 2011/61 / EU irányelv (MiFID II) szerint marketingkommunikációnak minősül, továbbá nem minősül befektetési tanácsadásnak vagy befektetési kutatásnak. A marketingkommunikáció nem befektetési ajánlás vagy információ, amely befektetési stratégiát javasol a következő rendeleteknek megfelelően, Az Európai Parlament és a Tanács 596/2014 / EU rendelete (2014. április 16.) a piaci visszaélésekről (a piaci visszaélésekről szóló rendelet), valamint a 2003/6 / EK európai parlamenti és tanácsi irányelv és a 2003/124 / EK bizottsági irányelvek hatályon kívül helyezéséről / EK, 2003/125 / EK és 2004/72 / EK, valamint az (EU) 2016/958 bizottsági felhatalmazáson alapuló rendelet (2016. március 9.) az 596/2014 / EU európai parlamenti és tanácsi rendeletnek a szabályozási technikai szabályozás tekintetében történő kiegészítéséről a befektetési ajánlások vagy a befektetési stratégiát javasló vagy javasló egyéb információk objektív bemutatására, valamint az egyes érdekek vagy összeférhetetlenség utáni jelek nyilvánosságra hozatalának technikai szabályaira vonatkozó szabványok vagy egyéb tanácsadás, ideértve a befektetési tanácsadást is, az A pénzügyi eszközök kereskedelméről szóló, 2005. július 29-i törvény (azaz a 2019. évi Lap, módosított 875 tétel). Ezen marketingkommunikáció a legnagyobb gondossággal, tárgyilagossággal készült, bemutatja azokat a tényeket, amelyek a szerző számára a készítés időpontjában ismertek voltak , valamint mindenféle értékelési elemtől mentes. A marketingkommunikáció az Ügyfél igényeinek, az egyéni pénzügyi helyzetének figyelembevétele nélkül készül, és semmilyen módon nem terjeszt elő befektetési stratégiát. A marketingkommunikáció nem minősül semmilyen pénzügyi eszköz eladási, felajánlási, feliratkozási, vásárlási felhívásának, hirdetésének vagy promóciójának. Az XTB S.A. nem vállal felelősséget az Ügyfél ezen marketingkommunikációban foglalt információk alapján tett cselekedeteiért vagy mulasztásaiért, különösen a pénzügyi eszközök megszerzéséért vagy elidegenítéséért. Abban az esetben, ha a marketingkommunikáció bármilyen információt tartalmaz az abban megjelölt pénzügyi eszközökkel kapcsolatos eredményekről, azok nem jelentenek garanciát vagy előrejelzést a jövőbeli eredményekkel kapcsolatban.


