Vir Biotechnology (VIR.US) announced today that the US government will buy an additional 600,000 doses of sotrovimab, its Covid-19 treatment developed together with British partner GlaxoSmithKline (GSK.UK). The extra doses will be delivered in the first quarter of 2022 to the US, which has an option to purchase more in the second quarter.
Including today's announcement, the two companies have agreements for the sale of roughly 1.7 million doses of sotrovimab worldwide. Sotrovimab was granted emergency use authorization by the Food and Drug Administration in May 2021 for treatment of mild to moderate COVID-19 in at-risk adults and children 12 and older. Preclinical data has shown treatment is effective against all COVID-19 variants, including delta and omicron.
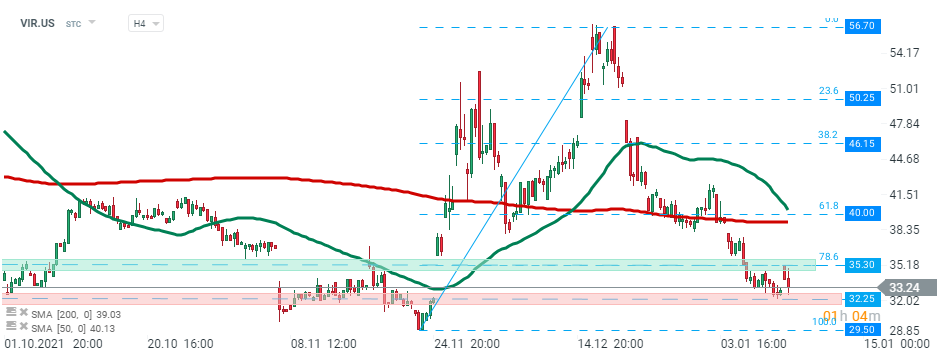 Vir Biotechnology (VIR.US) stock rose nearly 9.0% in premarket, however later in the session bullish momentum faded away. Buyers failed to break above the local resistance zone at $35.30 which is marked with 78.6% Fibonacci retracement of the last upward wave and price pulled back to recent lows at $32.25. Source: xStation5
Vir Biotechnology (VIR.US) stock rose nearly 9.0% in premarket, however later in the session bullish momentum faded away. Buyers failed to break above the local resistance zone at $35.30 which is marked with 78.6% Fibonacci retracement of the last upward wave and price pulled back to recent lows at $32.25. Source: xStation5

NFP preview

Economic calendar: NFP data and US oil inventory report 💡

Daily summary: Weak US data drags markets down, precious metals under pressure again!
Datadog in Top Form: Record Q4 and Strong Outlook for 2026
This content has been created by XTB S.A. This service is provided by XTB S.A., with its registered office in Warsaw, at Prosta 67, 00-838 Warsaw, Poland, entered in the register of entrepreneurs of the National Court Register (Krajowy Rejestr Sądowy) conducted by District Court for the Capital City of Warsaw, XII Commercial Division of the National Court Register under KRS number 0000217580, REGON number 015803782 and Tax Identification Number (NIP) 527-24-43-955, with the fully paid up share capital in the amount of PLN 5.869.181,75. XTB S.A. conducts brokerage activities on the basis of the license granted by Polish Securities and Exchange Commission on 8th November 2005 No. DDM-M-4021-57-1/2005 and is supervised by Polish Supervision Authority.


