Pfizer and BioNtech announced that their coronavirus vaccine candidate showed promising results in the third phase of clinical trials. Below you can find a list of key facts about vaccine.
-
Large scale trial: 43,000 participants
-
Participants were applied either vaccine candidate or placebo
-
Participants received two doses of vaccine
-
10% of participants who received vaccine contracted Covid-19
-
Vaccine shows 90% efficiency in preventing Covid-19
-
Companies plan to produce up to 50 million doses this year and up to 1.3 billion doses in 2021
-
Pfizer and BioNTech approached US regulator for emergency approval
Release triggered a massive market reaction with DE30 jumping from 12,675 to 13,300 pts and US500 moving from 3,550 pts to a fresh, intraday all-time high near 3,650 pts. Oil and industrial metals also experienced a massive jump while gold pulled back. JPY and CHF lost ground while commodity currencies like AUD, NZD and CAD surged. A closer look at the stock market shows that stocks from the "old economy" were top gainers on the news while pandemic winners slipped. Airlines, industrial and entertainment companies rallied while techs and biotechs pulled back.
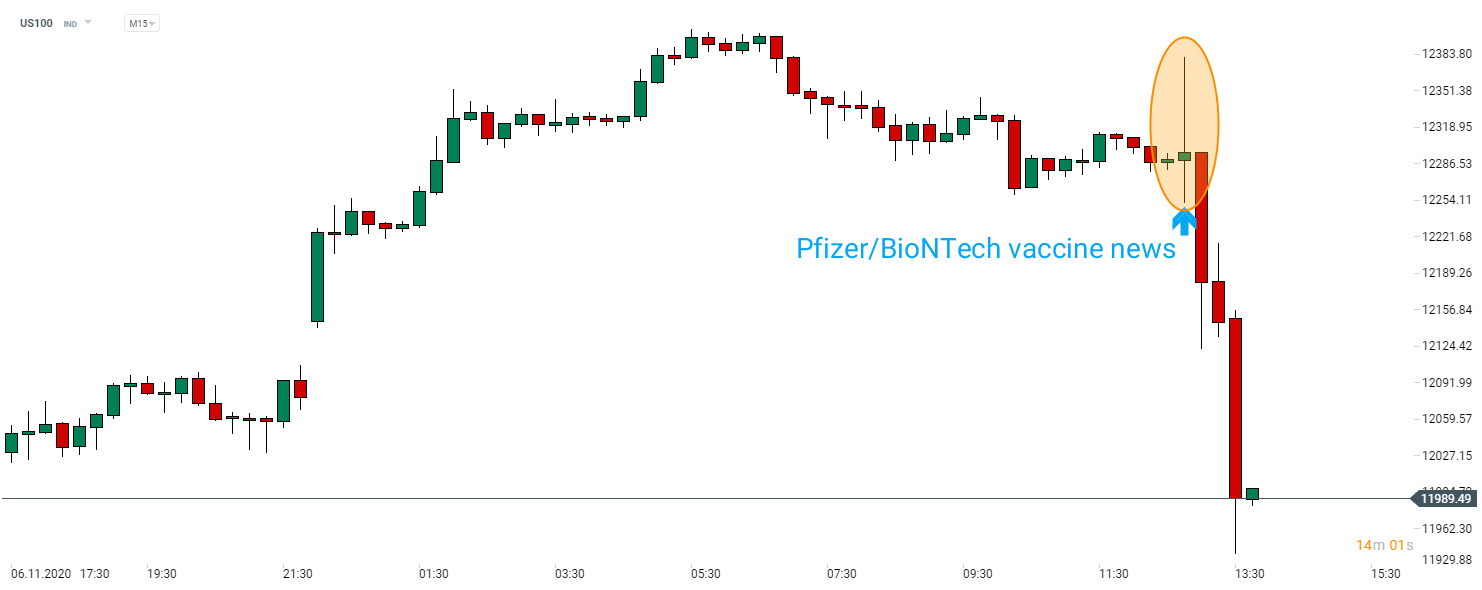 Tech stocks can be seen as losers of Pfizer/BioNTech vaccine announcement. US100 is trading 2.5% lower compared to pre-news levels. Source: xStation5
Tech stocks can be seen as losers of Pfizer/BioNTech vaccine announcement. US100 is trading 2.5% lower compared to pre-news levels. Source: xStation5

Wall Street extends gains; US100 rebounds over 1% 📈

Market wrap: Novo Nordisk jumps more than 7% 🚀

Takaichi’s party wins elections in Japan – a return of debt concerns? 💰✂️

The Week Ahead
This content has been created by XTB S.A. This service is provided by XTB S.A., with its registered office in Warsaw, at Prosta 67, 00-838 Warsaw, Poland, entered in the register of entrepreneurs of the National Court Register (Krajowy Rejestr Sądowy) conducted by District Court for the Capital City of Warsaw, XII Commercial Division of the National Court Register under KRS number 0000217580, REGON number 015803782 and Tax Identification Number (NIP) 527-24-43-955, with the fully paid up share capital in the amount of PLN 5.869.181,75. XTB S.A. conducts brokerage activities on the basis of the license granted by Polish Securities and Exchange Commission on 8th November 2005 No. DDM-M-4021-57-1/2005 and is supervised by Polish Supervision Authority.


