The biotech company Novavax (NVAX.US) has risen more than 20% during today's trading session on Wall Street, following news from the head of the European Medicines Agency (EMA). As reported by Reuters, the head of the EMA said that a vaccine being developed by US company Novavax will be authorized "in the very near future". The company's vaccine has so far been authorized in Indonesia and the Philippines. During clinical trials in the US, UK and Mexico, the NVX-CoV2373 vaccine has demonstrated over 90% efficacy. The company announced on 2 December that it is able to produce Omicron variant vaccines as early as January 2022.
Start investing today or test a free demo
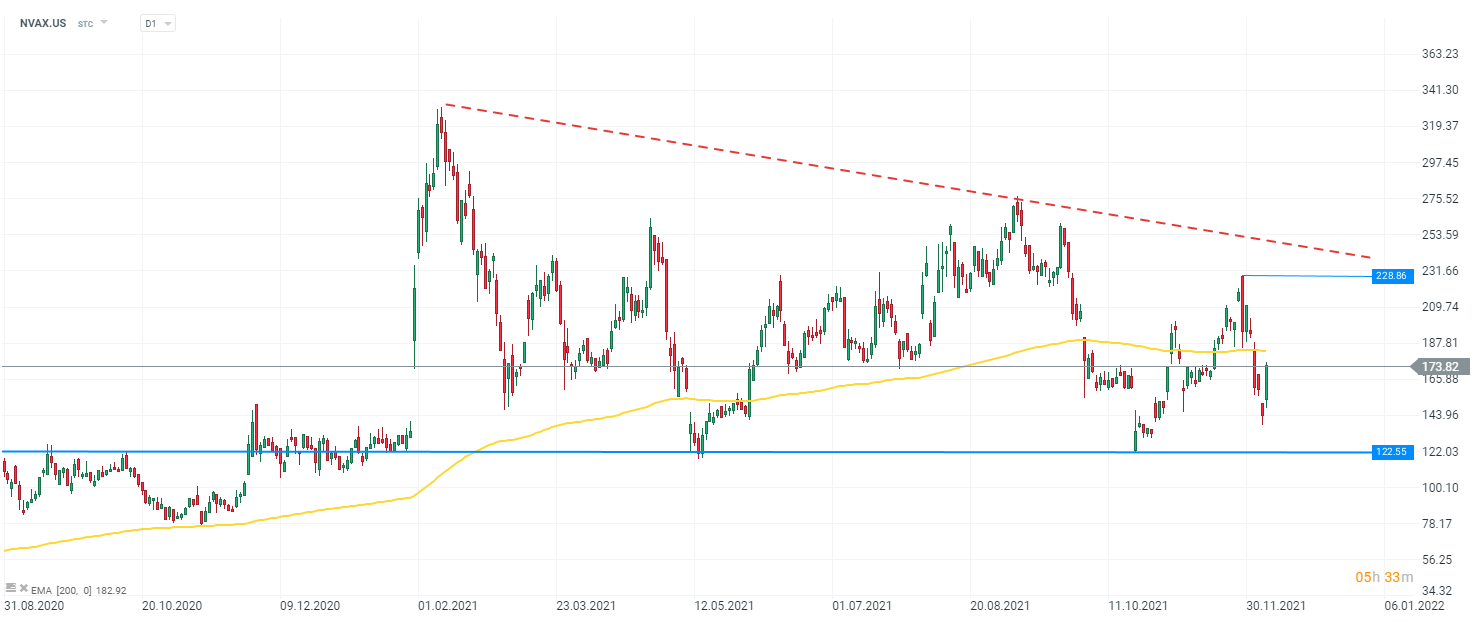
Open account Try demo Download mobile app Download mobile appNovavax (NVAX.US) stock, D1 interval. From the perspective of technical analysis, the company is moving in a descending triangle formation. Currently, the price is testing the resistance set by the 200 EMA (yellow line). If the demand side manages to break above this limit, it will be possible to test the local peak near $229 per share. If the upside is stopped, the key support remains the lower limit of the descending triangle formation at $122.5 usd per share. Source: xStation 5
This content has been created by XTB S.A. This service is provided by XTB S.A., with its registered office in Warsaw, at Prosta 67, 00-838 Warsaw, Poland, entered in the register of entrepreneurs of the National Court Register (Krajowy Rejestr Sądowy) conducted by District Court for the Capital City of Warsaw, XII Commercial Division of the National Court Register under KRS number 0000217580, REGON number 015803782 and Tax Identification Number (NIP) 527-24-43-955, with the fully paid up share capital in the amount of PLN 5.869.181,75. XTB S.A. conducts brokerage activities on the basis of the license granted by Polish Securities and Exchange Commission on 8th November 2005 No. DDM-M-4021-57-1/2005 and is supervised by Polish Supervision Authority.
