Merck (MRK.US) stock jumped over 9% during today's session after drugmaker announced its new antiviral drug molnupiravir lower the risk of death or hospitalization by 50% for Covid patients. Experts say antiviral pills could be the last tool needed to beat back Covid-19.
Only 7.3% of patients who took the pill were hospitalized according to Phase 3 study results and none of them died. Meanwhile 14.1% of placebo recipients had to be hospitalized. Eight patients in the placebo group died. The pharmaceutical company plans to file for emergency use authorization. Today's news negatively affected other pharma-giants stocks. Pfizer-partner BioNTech (BNTX.US) and Novavax (NVAX.US) at one point fell 13% and 18%, respectively.
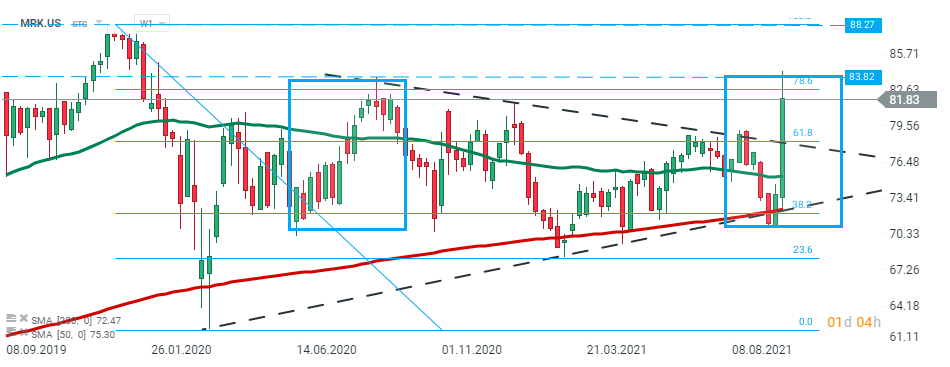 Merck (MRK.US) stock launched today's session sharply higher and broke above the lower limit of the triangle formation. Currently price is testing major resistance at $83.80 which is marked with upper limit of the 1:1 structure and 78.6% Fibonacci retracement of the last downward wave. Should a break higher occur, then the upward move may accelerate towards resistance at $ 88.27. However, if sellers will manage to halt advances here, then another downward impulse towards support at $78.35 may be launched. Source: xStation5
Merck (MRK.US) stock launched today's session sharply higher and broke above the lower limit of the triangle formation. Currently price is testing major resistance at $83.80 which is marked with upper limit of the 1:1 structure and 78.6% Fibonacci retracement of the last downward wave. Should a break higher occur, then the upward move may accelerate towards resistance at $ 88.27. However, if sellers will manage to halt advances here, then another downward impulse towards support at $78.35 may be launched. Source: xStation5

Daily summary: Silver plunges 9% 🚨Indices, crypto and precious metals under pressure

Does the current sell-off signal the end of quantum companies?

Howmet Aerospace surges 10% after earnings reaching $100 bilion market cap 📈

US Open: Cisco Systems slides 10% after earnings 📉 Mixed sentiments on Wall Street
This content has been created by XTB S.A. This service is provided by XTB S.A., with its registered office in Warsaw, at Prosta 67, 00-838 Warsaw, Poland, entered in the register of entrepreneurs of the National Court Register (Krajowy Rejestr Sądowy) conducted by District Court for the Capital City of Warsaw, XII Commercial Division of the National Court Register under KRS number 0000217580, REGON number 015803782 and Tax Identification Number (NIP) 527-24-43-955, with the fully paid up share capital in the amount of PLN 5.869.181,75. XTB S.A. conducts brokerage activities on the basis of the license granted by Polish Securities and Exchange Commission on 8th November 2005 No. DDM-M-4021-57-1/2005 and is supervised by Polish Supervision Authority.


