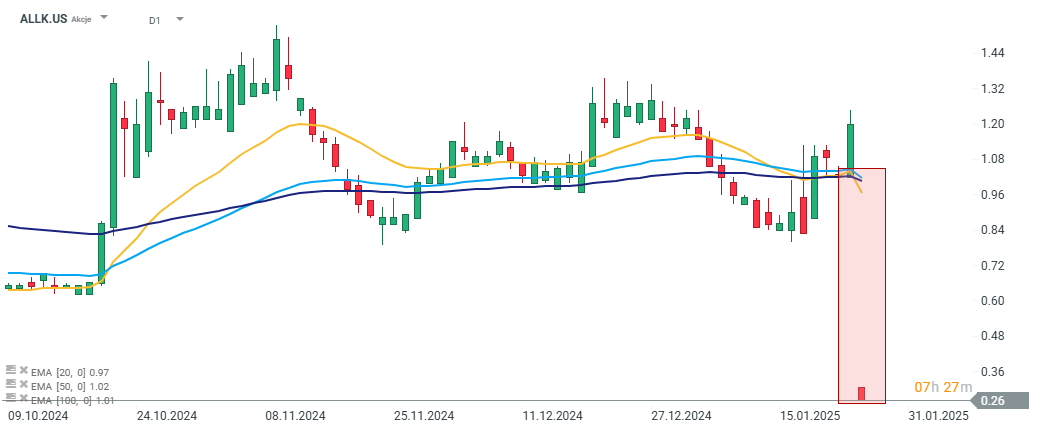
Allakos (ALLK.US) is down more than 77% today after releasing disappointing results from clinical trials of its drug AK006. The drug was intended to treat Chronic Spontaneous Urticaria (CSU), but the phase 1 trial results did not demonstrate sufficient therapeutic activity. As a result, the company has decided to abandon the project, which was one of its leading programs. Following the discontinuation of the project, the company announced it will lay off 75% of its workforce and will begin a review of available strategies to find a new alternative for its future operations.
Source: xStation

Arista Networks closes 2025 with record results!

AI scare trade broadens out as we wait for key inflation update

Daily summary: Silver plunges 9% 🚨Indices, crypto and precious metals under pressure

Does the current sell-off signal the end of quantum companies?
This content has been created by XTB S.A. This service is provided by XTB S.A., with its registered office in Warsaw, at Prosta 67, 00-838 Warsaw, Poland, entered in the register of entrepreneurs of the National Court Register (Krajowy Rejestr Sądowy) conducted by District Court for the Capital City of Warsaw, XII Commercial Division of the National Court Register under KRS number 0000217580, REGON number 015803782 and Tax Identification Number (NIP) 527-24-43-955, with the fully paid up share capital in the amount of PLN 5.869.181,75. XTB S.A. conducts brokerage activities on the basis of the license granted by Polish Securities and Exchange Commission on 8th November 2005 No. DDM-M-4021-57-1/2005 and is supervised by Polish Supervision Authority.


