Cartesian Therapeutics (RNAC.US) has announced that an experimental cell therapy it is developing has been successful in a mid-stage clinical trial of people with the rare autoimmune disease myasthenia gravis.
According to the company, 10 of the 14 participants who received the therapy and, according to the study design, were evaluated for efficacy, had a 5-point or greater reduction in disease severity measures after three months of follow-up. By comparison, three of the 12 people receiving placebo met this requirement.
Despite this news, however, the company's shares are losing nearly 31% today. Analysts on the conference call focused on the company's decision to exclude nine volunteers from the efficacy analysis, which in the eyes of many undermined the statistical effectiveness of the studies. The company acknowledged that the decision lowered the statistical power of the study, but that it was required to ensure equal study conditions.
Moreover, the company is announcing a private placement of equity financing worth $130 million, thereby selling a total of 3,563,247 common shares and 2,937,903 convertible non-voting series preferred shares at $20.00 per share.
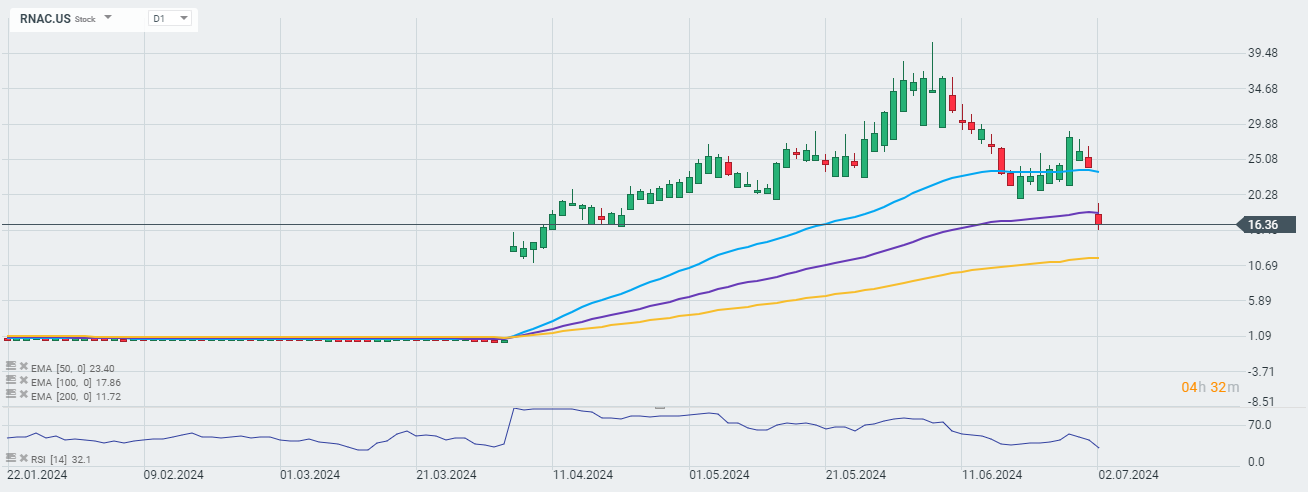 Source: xStation
Source: xStation

Daily summary: Weak US data drags markets down, precious metals under pressure again!
Datadog in Top Form: Record Q4 and Strong Outlook for 2026

US Open: Wall Street rises despite weak retail sales

Coca-Cola Earnings: Will the New CEO Withstand the Pressure?
This content has been created by XTB S.A. This service is provided by XTB S.A., with its registered office in Warsaw, at Prosta 67, 00-838 Warsaw, Poland, entered in the register of entrepreneurs of the National Court Register (Krajowy Rejestr Sądowy) conducted by District Court for the Capital City of Warsaw, XII Commercial Division of the National Court Register under KRS number 0000217580, REGON number 015803782 and Tax Identification Number (NIP) 527-24-43-955, with the fully paid up share capital in the amount of PLN 5.869.181,75. XTB S.A. conducts brokerage activities on the basis of the license granted by Polish Securities and Exchange Commission on 8th November 2005 No. DDM-M-4021-57-1/2005 and is supervised by Polish Supervision Authority.


