Bristol Myers (BMY.US) shares are up 4% ahead of the week’s final session on Wall Street after the drugmaker won approval from the U.S. Food and Drug Administration for its novel drug Cobenfa as a treatment for schizophrenia in adults. Analysts have commented that the FDA approval could help Bristol approve its acquisition of Karuna Therapeutics, which would theoretically expand BMY’s sales capabilities.
The FDA-approved drug is notable for being the first antipsychotic drug to target cholinergic receptors rather than dopamine receptors, which could be safer for patients in some situations. It is the first approved drug for schizophrenia in 30 years.
Start investing today or test a free demo
Create account Try a demo Download mobile app Download mobile app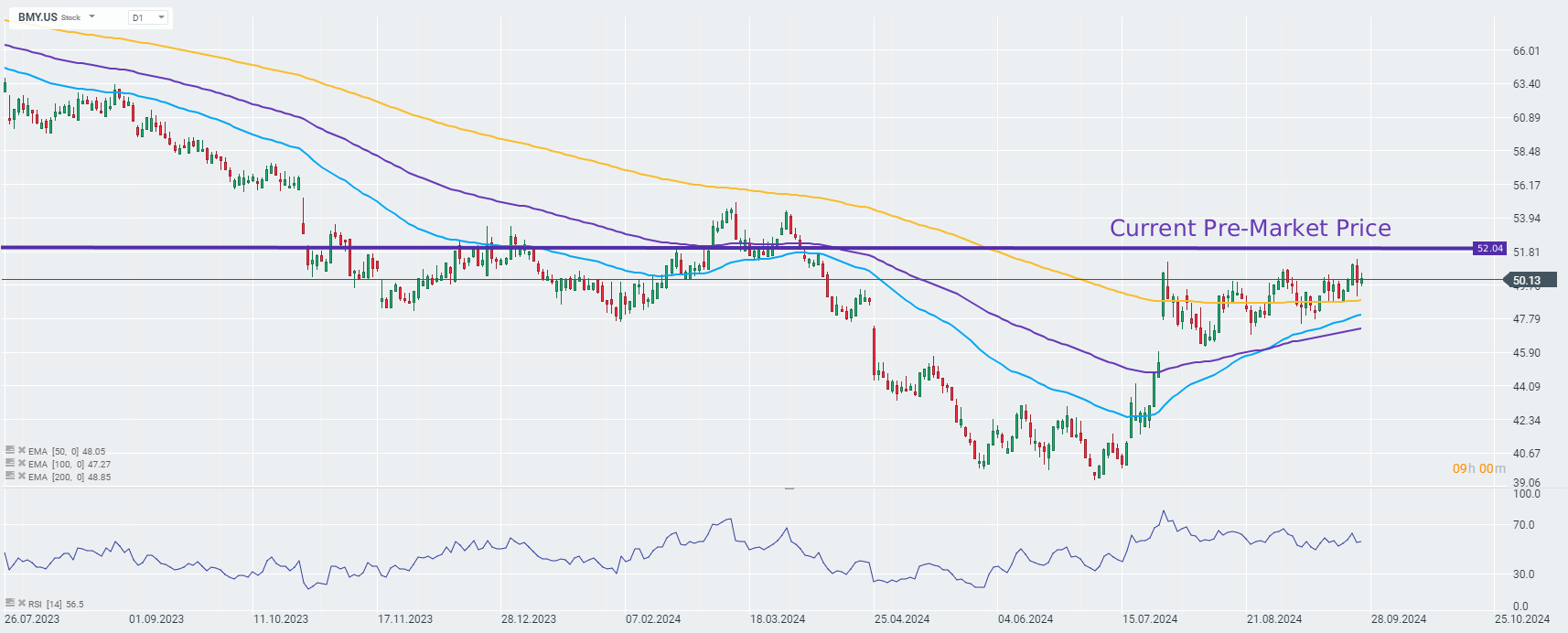
The stock is gaining ahead of the open and is trading well above the 200-day EMA (golden line on the chart), which could define the overall trend in the stock over the long term. Source: xStatio
