Cartesian Therapeutics (RNAC.US) has announced that an experimental cell therapy it is developing has been successful in a mid-stage clinical trial of people with the rare autoimmune disease myasthenia gravis.
According to the company, 10 of the 14 participants who received the therapy and, according to the study design, were evaluated for efficacy, had a 5-point or greater reduction in disease severity measures after three months of follow-up. By comparison, three of the 12 people receiving placebo met this requirement.
Start investing today or test a free demo
Open real account TRY DEMO Download mobile app Download mobile appDespite this news, however, the company's shares are losing nearly 31% today. Analysts on the conference call focused on the company's decision to exclude nine volunteers from the efficacy analysis, which in the eyes of many undermined the statistical effectiveness of the studies. The company acknowledged that the decision lowered the statistical power of the study, but that it was required to ensure equal study conditions.
Moreover, the company is announcing a private placement of equity financing worth $130 million, thereby selling a total of 3,563,247 common shares and 2,937,903 convertible non-voting series preferred shares at $20.00 per share.
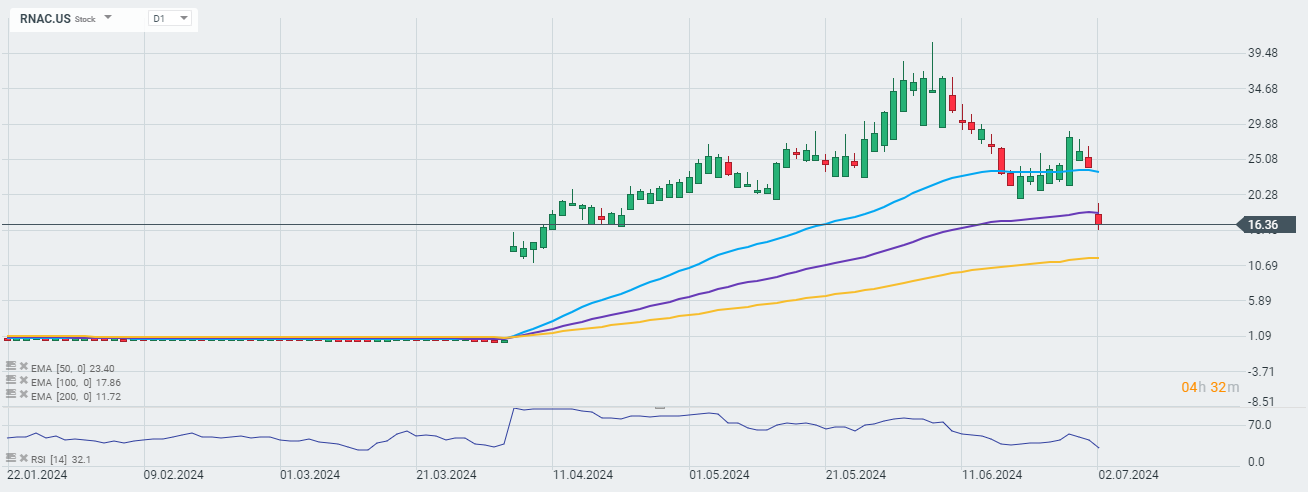 Source: xStation
Source: xStation
